It offers a way to convert heat into work through the implementation of the circular (periodic) process is characterized in that the only result of which is the production of works at the expense of cooling (a decrease in the internal energy) of thermal reservoir in the systems which are in force fields.
The idea of the proposed method consists in the fact that the second law of thermodynamics is not absolute (i.e., unlimited fair), and has certain limitations. So, for example, thermodynamic system in order for it would be possible to apply the second law must meet certain requirements, namely, to be closed, be of a certain size, etc.
Thus the second law is not applicable in open thermodynamic systems, in the microworld (the "Brownian motion" this law is not applicable) in megaworld (the hypothesis of the thermal death of the universe is not supported), etc. Consequently, outside the limits of applicability of the second law possible way of converting heat into work in a circular (periodic) process, which uses only a single heat reservoir at the same temperature, and where all system messages from the outside heat is converted into useful work without removal of the heat in the heat sink (fridge). Such, for example, may be thermodynamic systems are in external force fields (gravitational, electrostatic, etc.), i.e. not closed (open) the thermodynamic system.
This method can be realized by different devices (systems), some of them are described below. Systems are in an external potential force field, such as the gravitational g. In these systems the entropy decreases because of the anti-entropic nature of the gravitational field, so they are not closed (isolated) systems in the sense of the second law of thermodynamics. When considering the operation of the minor losses (on friction, the irreversibility and etc.) are considered negligibly small and is not taken into account.
The idea of the work of the following devices is that the amount of heat needed to heat (cooling) of a rigid body depends on the point of attachment of the body, because the heat is spent not only on the change of the internal energy, but also to change the height of the centre of gravity of a rigid body.
For example, consider the system (pic.1) running continuously (loop) consisting of a thermally insulated volume 1, heat engine 2, heat pump 3 and solid body 4. The temperature outside is insulated volume Tout, and within Tint, and Tout > Tint. If you run the thermal machine then it will start working and heater she serves as the external environment, and refrigerator internal volume, the thermal machine consumes outside the warmth of Qhm with the temperature of the Tout, and gives the inner space of the warmth of Qh with the temperature of the Tint. To maintain the internal temperature of the Tint at a level lower than the Tout is a heat pump, which works in reverse cycle and heat pumps Qc with the lower temperature level Tint in the upper temperature level Tout.
The key element of this system is a solid body. Let's consider the work of a rigid body (pic.2):
Suppose that initially the solid body is at a temperature of Tint (pic.2a). When heating a solid body warmth Qh from the refrigerator thermal machine, as a result of thermal expansion, its height and the height H of the centre of gravity c.g. of the increases (pic.2b). Then the upper end of the rigid body motionless, prescribe, after which cooled heat pump, selecting the warmth of Qc to the initial temperature Tint in this, and its height again becomes the initial (pic.2c). As a result of these actions of the body's center of gravity rises on the value of dH. After cooling of the solid body is immersed in the initial position (pic.2a) , and the cycle is closed.
According to the first law of thermodynamics the amount of heat Qs reported a solid body as well:
Qs = Qh + Qc = (dUh + Ah) + (-dUc + Ac) = Ah + Ac = m*g*dH,
where Qh, Qc - quantity of heat, reported a solid body in the heating and cooling respectively;
dUh, dUc - the change of the internal energy of a solid body in the heating and cooling respectively (equal);
Ah, Ac - the work done by solid body against external forces (gravity) when heating and cooling respectively;
m - the mass of the rigid body;
g - the acceleration of free fall.
Total useful work As when lowering the centre of gravity of a rigid body:
As = m*g*dH.
Thus Qs = As.
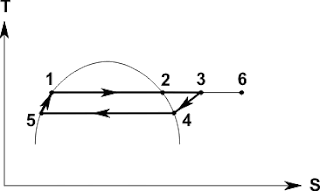
Let's consider the work of the above mentioned system in the TS-diagram (temperature-entropy) (pic.3):
Originally, the system is at a temperature of Tint (point 1). The heating of the rigid body is in the process of the 1-2 due to the selection of warmth Qh the refrigerator thermal machine (which works on cycle 4-2-1) heater which provides the warmth of Qhm at a temperature of Tout. Cooling of a rigid body is in the process of the 2-3 due to the transfer of heat Qc heat pump (which works on cycle 5-3-2) who gives the warmth of Qhp at a temperature of Tout. From the TS-diagram it is evident that Qhm > Qhp, i.e. heat, dedicated to the heat pump is completely absorbed heat machine, and missing the warmth comes from the outer environment, respectively the work of heating machines Ahm more work needed to drive a heat pump Ahp.
Total useful work A and the number of reported heat Q as a result of the cycle:
A = As + Ahm + (-Ahp) = Tout*dS,
Q = Qhm + (-Qhp) = Tout*dS,
where dS - the total change in entropy of the system.
Thus A = Q.
The work of a solid body in the system can be changed (pic.4):
Suppose that the length of the rigid body substantially more cross-section (diameter) so that the change of the sizes of a rigid body with a thermal expansion occurs mainly in length. Suppose that initially the solid body is at a temperature of Tint (pic.4a). When heating a solid body warmth Qh from the refrigerator thermal machine, as a result of thermal expansion, its length (height) and the height of the centre of gravity increases (pic.4b). Then the solid body turns 90 degrees around the center of gravity (pic.4c) then cooled heat pump, selecting the warmth of Qc to the initial temperature Tintin this, and its length again becomes the initial (pic.4d). After which a solid body again turn by 90 degrees around the center of gravity (pic.4e). As a result of these actions of the body's center of gravity rises on the value of dH. After cooling of the solid body is immersed in the initial position (pic.4a), and the cycle is closed.
According to the first law of thermodynamics the amount of heat Qs reported a solid body as well:
Qs = Qh + Qc = (dUh + Ah) + (-dUc) = Ah = m*g*dH,
where Qh, Qc - quantity of heat, reported a solid body in the heating and cooling respectively;
dUh, dUc - the change of the internal energy of a solid body in the heating and cooling respectively (equal);
Ah - the work done by solid body against external forces (gravity) when heated;
m - the mass of the rigid body;
g - the acceleration of free fall.
Total useful work As when lowering the centre of gravity of a rigid body:
As = m*g*dH.
Thus Qs = As.
TS-diagram of this system is similar to shown in pic.3.
The idea of the next device consists in the fact that the heated and cooled liquid has a different density, and, consequently, the pillars of the liquid of the same height, but different temperature will have a different weight.
The device (system) consists of thermal insulation 1, lift 2 and the surge 3 pipes height H, two heat exchangers 4 and 5, and hydraulic machines 6 forming a closed circuit filled with a liquid, heating machines 7, the heat pump 8.
The device operates in the following way.
The liquid is heated in the heat exchanger 5 due to the selection of warmth in the cooler heat machine 7, it rises to a height of H on the lift tube 2 to the heat exchanger 4, where it is cooled up to the temperature due to the transfer of heat of thermal pumps 8. After cooling of the liquid falls on the surge pipe 1. Due to the density difference of heated and chilled fluid appears at the bottom of the pressure difference, which is processed in the hydraulic machine 6, transformed into useful work. Then the liquid enters the heat exchanger 5 and the cycle is closed. The heat capacity of the fluid little depends on the pressure, but all the same dependence is, therefore, in the lower heat exchanger 5 the quantity of heat consumed more than in the upper heat exchanger 4 is allocated, i.e. Qhm > Qhp, i.e. heat, dedicated to the heat pump is completely absorbed heat machine, and missing the warmth comes from the environment, respectively the work of the thermal machine, more work needed to drive a heat pump.
Calculation of the total amount of heat notified to the system and useful work the same as the device with hard working body considered above.
TS-diagram of this system is similar to shown in pic.3.
The idea of the work of the following devices is that their gaseous working body operates a variable force field, which performs the role of "Maxwell's demon" sorting molecules on the velocity.
This is how Sivukhin D. V. (General course of physics: handbook: For institutions of higher education. In 5 t. Thermodynamics and molecular physics. - 5-e izd., corr. - M.: Fizmatlit, 2005, 544 p.) a similar experience on the page 274: "...thermally insulated vessel with an ideal gas is suspended on a thread in the gravity field. Because of the action of the force of gravity density of the gas at the bottom of the vessel more than at the top. Thread burn through, and the vessel falls freely. Assuming that during the fall of time to be established, thermodynamic equilibrium, determine the equilibrium temperature of the gas, which it is established in the fall.
D e c i s i o n. The gas temperature will not change. In a free fall of the gas is in a state of weightlessness. The initial state of the nonequilibrium density at the top of less than at the bottom. However, the average kinetic energy of molecules everywhere the same. During the transition to the equilibrium state density aligned. But the full kinetic energy of the molecules of the gas, which determines its temperature, will remain unchanged. The experience is similar to the well-known experiment of Gay-Lussac with the expansion of gas into a vacuum ... "
The device (system) (pic.6) consists of a vessel constant volume with gas 1, mobile air-tight walls 2, the gas-expansion machine 3 (expander).
The device operates in the following way.
The initial state of the gas unbalanced because of the actions of the force of gravity, density and pressure of it at the bottom of the vessel more than at the top (according to the law of distribution of the Boltzmann), however, the average kinetic energy of molecules (which determines the temperature of the gas) everywhere the same (pic.6a). Then the vessel with gas share a partition 2 in the upper and lower volumes, which can be provided only through the expansion of the 3. After that the device falls freely (pic.6b). In a free fall of the gas is in a state of weightlessness and goes to the equilibrium state, изотермически expanding in gas-expansion unit 3 by making work an equivalent number of input from the outside heat. During the transition to the equilibrium state of the density and pressure of gas everywhere is balanced. Further, during the fall, the wall opened, and the device is inhibited, the gas gets back to the initial non-equilibrium state (pic.6c). During braking, the gas temperature does not change because unchanged full kinetic energy of the molecules of the gas, which determines its temperature. The force of gravity changes only the concentration of molecules at different altitudes in the container with the gas, but not the temperature. The device then raise the initial height (pic.6a), and the cycle is closed.
Heat Q in the loop is applied only in the isothermal process in gas-expansion machine, completely transformed into the equivalent her work:
Q = Ae,
where Q – quantity of heat, reported by the system from the outside;
Ae – expander work.
The total work A cycle:
A=Alow – Aup + Ae = Ae,
where Alow, Aup – of lowering and hoisting systems, respectively (equal).
Thus A = Q.
The following device (system) (pic. 7) consists of a vessel with a gas and a piston moving horizontally in it. The system is thermally insulated, heat insulation and open only during the isothermal process.
The work of the TS-diagram (temperature-entropy) is shown in pic 8.
The device operates as follows.
Initially the gas is in a compressed state at T (pic.7a) (pic.8, point 1), its center of gravity, because of the effect of gravity in accordance with the distribution law Boltzmann constant, at an altitude h from the bottom of the vessel wall. The device then begins to fall freely from a height H. Free-fall gas is in a state of weightlessness and proceeds to an equilibrium state, the density and pressure of the gas is everywhere even out, and the total kinetic energy of gas molecules, which determines its temperature remains unchanged. The gas (during fall) expands isothermally at first (pic.7b) (pic.8, the process 1-2), performing work equivalent to the amount of the input from the outside heat Q, and then isoentropic (pic.7c) (pic.8, the process 2-3), with the result that its temperature is lowered to a temperature T3. After that inhibit device (pic.7d). During braking the gas temperature does not change because unchanged total kinetic energy of gas molecules, which determines its temperature. The force of gravity changes only the concentration of molecules at different heights in the vessel with gas, but not temperature. Since the gas temperature (after isentropic expansion) has fallen, his center of gravity, because of the effect of gravity in accordance with the law of the Boltzmann distribution, is now located at lower altitude h3 from the bottom of the vessel wall (pic.7d). The gas is then compressed, and the process will take place on the polytropic (pic.7e) (pic.8, the process 3-1), as the work of compression is divided into two parts: one part is spent on increasing the kinetic energy of gas molecules, ie internal energy (and temperature) of the gas, the other part is spent on increasing the potential energy of the gas molecules, ie to raise the center of gravity height of the gas mass c h3 to h. As a result of a polytropic compression temperature T and height h center of gravity of the gas from the bottom of the vessel wall once again become the original. Then pick up the device to its original state (pic.7a) and the cycle closes.
The total work A loop:
A = Ait + Aie – Ap + Alow – Aup = Ait,
where Ait – working gas in an isothermal process;
Aie – working gas in isoentropic process;
Ap – polytropic gas work in process;
Alow = m*g*(H + h – h3) – lowering the mass of working gas;
Aup = m*g*H – work lifting a mass of gas.
m – the mass of gas;
g - acceleration of free fall;
The total heat Q loop:
Q = Qit,
where Qit – the amount of heat imparted from outside the system in an isothermal process;
Thus heat Q in the loop is supplied only to an isothermal process is completely transformed into an equivalent job, ie Q = A.
The following device (system) (pic.9) consists of three vessels of the upper 1, the lower 2, the average 3 with gas pistons and moving horizontally in them interconnected with pipes 4. The upper and lower vessels are at a distance (height) H from one another, and the average vessel can be moved in height. For simplicity of reasoning and calculation, we assume the volume of the tube small compared to the volume of blood vessels, and the vertical size (diameter) of vessels much smaller than the height H. The system is thermally insulated, heat insulation and open only during the isothermal process.
TS-diagram of this system is similar.
The device operates as follows.
Initially the gas is at T (pic.9a) (pic.8, point 1), the total mass of gas m is distributed between the upper and lower communicating vessels (average vessel still empty). The center of gravity of the system of two communicating vessels with a gas top and bottom, because of the effect of gravity in accordance with the law of the Boltzmann distribution, at an altitude h from the bottom of the vessel. The mass of gas and pressure in the upper vessel m1, p1 lower m2, p2, respectively, naturally m = m1 + m2. Then all of the gas (isothermally at a temperature T) push the pistons out of the upper and lower vessels by connecting pipe in the middle vessel, located at an altitude of h (pic.9b) with m3 = m1 + m2 = m, and p3 equal to the pressure at this altitude in accordance with the law of the Boltzmann distribution (relative p1). As a result of the isothermal process is performed (spent), the work and released (absorbed) heat:
A3 - A1 - A2 = 0 and Q1 - Q2 = 0,
where A3 = p3*v3 – working gas when entering the middle vessel;
A1 = p1*v1 – work ejection of gas from the bottom of the vessel;
A2 = p2*v2 – work ejection of gas from the top of the vessel;
v1, v2, v3 – gas volumes in the respective vessels;
Q1 = m1*g*h – heat absorbed by the mass of gas at the bottom of the vessel picked up at the height of h;
Q2 = m2*g*(H – h) – heat released gas mass of the upper vessel by lowering the height of h;
g - acceleration of free fall;
Thus as a result of pumping gas into the middle vessel to the height of the center of gravity h, no thermodynamic changes in the system does not, and all the gas is concentrated in a single vessel (pumping can be accomplished in other ways, such as raising lower vessel to the height of the center of gravity h, and the upper vessel lowered to the same height, and then siphon all the gas in the middle vessel at a pressure p3, the result is the same). The gas expands at an average vessel initially isothermal (pic.9c) (pic.8, the process 1-2), performing work equivalent to the amount of the input from the outside heat Q, and then isoentropic (pic.9c) (pic.8, the process 2-3), with the result that its temperature is lowered to a temperature T3. Then, the average vessel is lowered from a height h to the height of h3 (height h3 is the center of gravity of the system of two communicating vessels with a gas top and bottom, with Let the average vessel in accordance with the law of the Boltzmann distribution for the temperature T3). The entire gas (isothermally at a temperature T3) push the piston from the middle of the vessel by connecting pipe in the upper and lower vessels (pic.9d), the processes occurring at the same time similar and diametrically opposed above, at the confluence of the gas in the middle vessel. Thus as a result of pumping gas in the upper and lower vessels (height of center of gravity of the system remained the same h3) is no thermodynamic changes in the system does not, and all the gas is concentrated in the upper and lower vessels at T3, while the mass of gas and pressure in connecting the upper and lower vessels correspond to the masses and pressures at these altitudes on the Law of the Boltzmann distribution, but at T3. The gas in connecting the upper and lower vessels to constrict. The compression process will take place on the polytrope (pic.8, process 3-1), as the work of compression is divided into two parts: one part is spent on increasing the kinetic energy of gas molecules, ie internal energy (and temperature) of the gas, the other part is spent on increasing the potential energy of the gas molecules, ie to raise the center of gravity mass of gas in the system of two vessels of height h3 to h. As a result of a polytropic compression temperature T and height h center of gravity of the gas from the bottom of the vessel again become a pioneer and the cycle closes.
The total work A loop:
A = Ait + Aie – Ap + Alow = Ait,
where Ait – the work of expansion of gas in an isothermal process;
Aie – work of gas expansion in isoentropic process;
Ap – work in polytropic gas compression process;
Alow = m*g*(h – h3) – lowering the average work of the vessel with the gas;
The total heat Q loop:
Q = Qit,
where Qit – the amount of heat imparted from outside the system in an isothermal process;
Thus heat Q in the cycle is supplied only to an isothermal process is completely transformed into an equivalent job, ie Q = A.
The following device (system) (pic.10) consists of a vessel with a gas and a piston moving horizontally in it. The system is thermally insulated, heat insulation and open only at the time of isothermal heating and cooling at constant volume (heat pump).
The work of the TS-diagram (temperature-entropy) is shown in pic.11.
The device operates as follows.
Initially the gas is at a low temperature (pic.11, point 1) center of gravity c.g. it, because of the effect of gravity in accordance with the law of the Boltzmann distribution, is at some height from the bottom of the vessel wall . The gas is then compressed (with an expenditure of work). The process will take place on the polytropic (pic.11, the process 1-2), as the work of compression is divided into two parts: one part is spent on increasing the kinetic energy of gas molecules, ie internal energy (and temperature) of the gas, the other part is spent on increasing the potential energy of the gas molecules, ie to raise the center of gravity mass of gas. As a result of a polytropic compression temperature and height of center of gravity of the gas from the bottom of the vessel wall increase. The gas expands isothermally in a container (with a performance of work) (pic.11, the process 2-3), with its center of gravity remains at the previous (higher) level (in accordance with the law of the Boltzmann distribution). At the same time is consumed by the heat from the outside Qi. The gas is then cooled at constant volume (pic.11 the process 3-1) heat pump (with an expenditure of work) to the initial low temperature and the cycle closes. Upon cooling, the process will take place on the polytropic as isochoric heat addition process must still take the heat released by lowering the center of gravity of the gas mass (ie the polytrope will be more hollow than the isochore v = const). In the process of cooling a heat pump (pic.11, the process 1-3-4) isolates in the external medium heat Qhp. From the TS-diagram shows that Qi > Qhp, ie the heat given to the heat pump is completely absorbed in an isothermal process, and the missing heat coming from the external medium, respectively, work in an isothermal process more work needed to drive the heat pump.
Total useful work A and the number of reported heat Q as a result of the cycle:
A = Ai – Ahp – Ap = m*g*h,
Q = Qi – Qhp = m*g*h,
where Ai, Qi – work and absorbed by the heat in the isothermal process, respectively;
Ahp, Qhp – the work of the heat pump and allocated them warmth, respectively;
Ap – work in a polytropic process;
m – the mass of gas;
g - acceleration of free fall;
h – raising the center of gravity of the gas.
Thus A = Q.
The idea of the next device is that the vessel is in Earth's gravitational field is present a mixture of light gas and fumes. In a mixture of pressure equal to the sum of partial pressures (Dalton's law of) lung liquid vapor and gas, and pressure drop (and the saturation temperature) with height (under the law of the Boltzmann distribution) is less than the vapor of the pure liquid (without a light gas).
Device (system) (pic.12) consists of a standpipe 1 and lift 2 pipes, heat exchangers 4 and 5, hydraulic machine 3, the heat pump 6, separator 8, thermal insulation 7. Standpipe 1 is filled with liquid, such as freon, a rising pipe 2 filled with a mixture of vapor and light liquid gas such as hydrogen. The upper part of the system is thermally insulated with thermal insulation 7 and is located at a considerable distance (height) H from the bottom (heat exchanger 4 and hydraulic machines 3).
The work of the TS-diagram (temperature-entropy) is shown in pic.13.
The device operates as follows.
Liquid from the separator 8 down on a standpipe 1 with height H and goes down to the hydraulic machine 3, where the change its potential energy is converted into work. In a hydraulic machine 3 enlarged to a liquid boiling pressure of the system at a given temperature and the heat exchanger 4, where it isothermally heat supply (pic.13, the process 1-2). In the process of boiling liquid is converted into dry saturated steam. After the heat exchanger 4 a pair of fluid mixed with a light gas, gas-vapor mixture and the resulting rises to a height H on the lift tube 2, where it isothermally heat supply . Gas-vapor mixture at raising its pressure decreases (according to the law of the Boltzmann distribution), and a pair of fluid contained in it are overheated (pic.13, the process 2-3). It should be noted that if a lift tube 2 would have been a pair of clear liquid, then the pressure at an altitude of H would be less (pic.13, the process 2-6). In a mixture of the same pressure is the sum of partial pressures (Dalton's law of) lung liquid and gas, and has a drop in pressure with height is much smaller. Then the gas-vapor mixture, which is at an altitude of H, the heat exchanger 5, where she was first cooled to the saturation liquid vapor in the gas (pic.13, the process 3-4), and then the dry saturated vapor liquid in the gas condenses in the process of removal of heat (pic.13, the process 4-5) heat pump 6. Further, the separator 8 is separated from the liquid and gas passing through the regenerative heat exchanger 5 (pic.13, the process 5-1) enters the standpipe antennas 1, closing the cycle, and the dry light gas regenerative heat exchanger in 5 and returns to the lift tube 2.
The total work A and the amount of heat Q in a loop:
A = A3 – A6,
Q = Q4 + Q2 – Q6,
where A3 – working hydraulic machine;
A6 – the work of the heat pump;
Q4 – the amount of heat imparted during the boiling liquid;
Q2 – amount of heat, gas-vapor mixture reported by lifting the tube;
Q6 – amount of heat cast by a heat pump.
Thus the higher pressure gas-vapor mixture at the inlet to the heat exchanger 5, the less work is spent heat pump 6, and the more total work A and equal to it consumed external heat Q.
The following device (system) is similar to the device in pic.1, only the main working body - vapor-fluid.
The device (system) (pic.14) consists of a thermally insulated volume 1, a heat engine 2, the heat pump 3 and a constant-volume vessel 4. The vessel is partially filled with liquid and partially vapor and the liquid has a size in length (height) is much larger than the size of its cross section.
The work of the TS-diagram (temperature-entropy) is shown in pic.15.
The device operates as follows.
Suppose the system is initially at a temperature Tint (point 1). The vessel is placed vertically, the liquid is at the bottom, a pair of fluid occupy the remaining volume from the top, center of gravity c.g. of the liquid-vapor is close near the center of gravity of the liquid itself, as fluid density is several orders higher than the density of its vapor. The liquid is then heated to a temperature Tout (process 1-2) through the selection of heat Qh in the refrigerator of a heat engine (which runs on a cycle 4-2-1) heater which receives heat Qhm at Tout. Fluid at the same time completely evaporates, turning into dry saturated steam (point 2 is located on the saturation line pair x = 1), center of gravity c.g. up systems and approaches the middle of the vessel. Next, turn the vessel horizontally (90 degrees around the center of gravity c.g.) and then cooled to initial temperature Tint (process 2-3) through the transfer of heat Qc heat pump (which runs on a cycle 2-5-3), which in turn gives warmth Qhp at Tout. Dry saturated steam is condensed into a liquid and the center of gravity c.g. of the vapor-liquid slightly lowered. By the way smaller than the cross section (diameter) of the vessel, the less and lowering the center of gravity c.g. system, and the process will approach isochore. After cooling, the vessel turned back upright in its original position, while lowering the center of gravity c.g. of the liquid-vapor work is done A, and the cycle closes. From the TS-diagram shows that Qhm > Qhp, ie heat cast heat pump is completely absorbed by the heat engine and the missing heat coming from the external medium, respectively, the work of a heat engine Ahm more work is needed to drive the heat pump Ahp.
Total useful work As and the number of reported heat Qs as a result of the cycle:
As = A + Ahm + (-Ahp) = Tout*dS,
Qs = Qhm + (-Qhp) = Tout*dS,
where A – work lowering the center of gravity c.g. of the liquid-vapor;
Ahm – the work of heat machines;
Ahp – the work of the heat pump;
dS - the total change in entropy of the system.
Thus As = Qs.
Of course, the vessel may be filled with not only the liquid and the vapor, but also liquid and gas. The work of such a device (system) is similar to the above.